|
The analytical instrument called a mass spectrometer was
developed in 1919 by Francis Aston in Cambridge, England.
A spectrometer is a spectrograph with measurement capabilities.
The importance of this technology was immediately recognized,
and Aston was awarded the Nobel Prize for his work in 1922.
So, what does a mass spectrometer do and how does it work?
Mass spectrometers permit the experimental determination
of atomic and molecular masses with great accuracy. Aston's
mass spectrometer had a precision of one part in 10,000, which
was sufficient for him to discover the isotopes of many elements.
Modern instruments are even more precise.
Ionization of sample
Courtesy: McREL |
Mass spectrometers operate under conditions of high vacuum,
typically torr. (A torr is a
unit of pressure, equal to 1.316 x
atmosphere. In comparison the pressure in outer space may
be in the order of torr.) Low-pressure
samples in the spectrometer's ionization chamber are exposed
to a beam of rapidly moving, energetic electrons shot
out of an electron gun. The samples can be a gaseous element
such as neon, the vapor of a solid or liquid element such
as mercury, or even the vapor of a molecule such as water
or methane. With modern technology it is possible to introduce
a wide variety of materials, including mixtures, into a mass
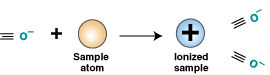
spectrometer. In the ionization chamber, the atom or molecule
hits or is hit by the accelerated electrons. During the collision
an electron is knocked out of the sample atom or molecule,
leaving it with a positive charge. In other words, positively-charged
gaseous ions are formed.
Ion accelerates in spectrometer chamber
Courtesy: McREL |
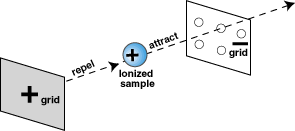
These newly-formed ions are then pushed out of the ionization
chamber by an electric field applied between two metal grids.
This is an application of Coulomb's law: the positively-charged
grid repels the positive ions and the negatively-charged grid
attracts the positive ions. This attraction and repulsion
both act in the same direction to give the ions a nudge (net
acceleration) toward the negatively-charged grid. The negative
grid, which is full of holes, allows the accelerated ions
to pass through it and leave the ionization chamber. The speeds
to which the ions can be accelerated by the electric field
are determined by their masses. Lighter ions reach higher
speeds than do the heavier ones.
Moving ion creates magnetic field
Courtesy: McREL |
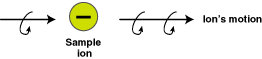
The accelerated beam of positively-charged ions generates
a magnetic field of its own, as do all moving electrically-charged
particles. The ion beam passes through an externally-applied
magnetic field. The magnetic field created by the beam of
moving charged particles interacts with the external magnetic
field. The net result is that the trajectory of each charged
particle is bent in a curve to an extent that depends on its
speed (and therefore its mass). If the beam of a mixture of
particles of different masses is allowed to hit a photographic
plate, the particles converge at different points, corresponding
to the different radii of their semicircular paths. Modern
mass spectrometers feed their results directly to computers
that do the analysis and produce a graph (spectrum). To learn
more about the mathematics behind mass spectrometry, read
"The Mathematics of Mass
Spectrometry."
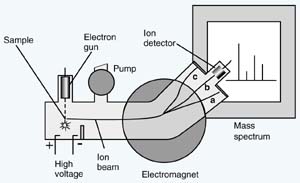
Diagram of Mass Spectrometer Courtesy:
McREL
Note. From Chemistry, Molecules, Matter and
Change, 3rd Ed., (p.9), by P. Atkins and L. Jones,
1997, New York: W. H. Freeman. |
There are many variations in the process of mass spectrometry,
but all of them are based on the principles outlined above.
Mass spectrometric techniques have played an important role
in science (particularly in chemistry). This historically
important technology is likely to play a major role in the
research phase of the Genesis project when the solar wind
samples are returned to Earth for analysis. By that time the
technology may be significantly improved over what is now
available
|
