|
The composition of the sun can be described in several ways.
By modern estimates, the composition by mass is: 71% H, 27%
He, and 2% other heavier elements. By number of atoms of a
given type, the sun's composition is: 91% H, 9% He, and 0.1%
other heavier elements. Hydrogen can mean either H atoms or
H molecules and context is needed
to make the meaning of the word clear. In the sun's core neither
hydrogen molecules nor neutral hydrogen atoms with one proton
in the nucleus and one orbital electron are present. The violent,
hot environment of the sun's center rips atoms apart into
their constituent pieces: protons, electrons, and other bare
atomic nuclei. Hydrogen in the sun's core is ionized,
a bare proton, represented by the symbol .
It is these protons that fuse together with the release of
energy.
What keeps the sun from exploding when all of those hydrogen
nuclei (protons) collide and fuse together? How has the sun
managed to ration its supply of hydrogen nuclei in such a
way as to preserve most of them for millions of years? The
answer to these questions is that the core, like the rest
of the sun, can be regarded as a gaseous body and analyzed
according to the principles of the Kinetic Molecular Theory
of Gases, which is well understood by researchers.
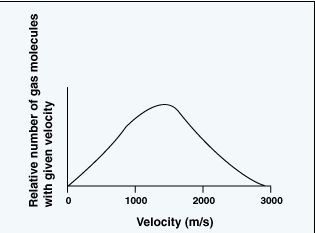
Velocity distribution of particles
Courtesy: McREL
|
In this model the temperature of a moving gas particle is
directly proportional to its velocity squared. Also, according
to this model there is a bell-shaped statistical distribution
of particle velocities in a sample of a gas, as shown here,
where the x-axis might represent either particle velocity
or particle temperature.
It should be clear that in a sample of gas a few particles
are almost motionless, while a few other particles are moving
at extraordinarily high velocities. In other words some particles
are cold (slow moving) and others are extraordinarily hot
(extremely fast moving). However, as indicated by the shape
of the curve, the largest portion of particles has a specific
velocity that corresponds to the average temperature of the
sample. So in the sun's core, even at its average "low" temperature,
there are present a relatively few extraordinarily hot protons
that are moving with much higher velocities than the "average"
proton.

Collision and repulsion of charged particles
Courtesy: McREL
|
Typically, the motion of particles of like charges is random,
resulting in collisions and repulsions. It almost seems as
if there were a barrier of some kind around each particle,
causing repulsion when they approach each other. Only a few
super-high-speed protons have enough kinetic energy to tunnel
through the electrostatic repulsion barrier and fuse together,
initiating the chain of events that ultimately provides the
energy from the sun's core. The average proton simply does
not have enough energy to tunnel through the barrier and fuse
with a collision partner. In other words the vast majority
of collisions do not lead to a fusion event.
Proton-proton fusion
In the mid-1930s, after the discovery of the neutron in 1932
and the construction of machines that could accelerate particles,
fusion reactions were demonstrated in earth-bound laboratories
and the essential correctness of the theoretical predictions
regarding fusion in the sun was established. It is now estimated
that at core temperatures, only one proton in 100 million
is hot enough to fuse during a collision. Put another way,
the reaction rate is so very slow that a specific proton would
require 14,000 billion years to find a suitably "hot" partner
with which to collide in a successful fusion event. Since
the sun is only about 4.5 billion years old, most of its protons
have not yet found a fusion partner.
How does proton-proton fusion work? First, two exceedingly
"hot" protons (hydrogen ions without electrons) collide. This
violent event results in the fusion of the two nuclei and
the formation of a deuteron, a positron, and a neutrino. This
event can be written conveniently in equation form, where
superscripts attached to elemental symbols represent mass
number and charge:

Formation of Deuterium
Courtesy: McREL
|
The symbols
represent a positron and a neutrino, respectively.
The deuteron, , differs from
a regular hydrogen nucleus in that it contains a neutron in
addition to a proton. In this reaction one of the protons
has been changed into a neutron, with the formation of a new
nucleus containing one proton and one neutron. The key transformation
can be written:
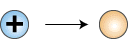
Proton becomes neutron
Courtesy: McREL
|
This equation cannot be correct as written, because
it does not account for the charge of the proton. On the left
side of the equation is a positive charge and on the right
side there is no charge. Note that the mass number is conserved.
What is indicated is the creation of a particle having a mass
of zero and a charge of plus one on the right side of the
equation. Thus we are introduced to the positron, ,
which is a positively charged electron: a particle of antimatter.
More correctly, this equation becomes:

Proton becomes neutron and positron
Courtesy: McREL
|
Now charge and mass number are conserved. However,
in natural reactions, momentum is also conserved. If a positron
speeds away, there must be something that flies out in the
opposite direction, since it has been determined that the
positron momentum is not balanced by recoil of the proton.
A neutrino, answering this requirement, is also emitted. The
neutrino is represented by the symbol n.
The next step in the so-called proton-proton cycle
that fuels the sun is the collision of another proton with
the deuteron that is formed, producing a helium nucleus
containing 2 protons and one neutron, symbolized as .
g
The symbol g represents
a gamma ray photon, which is required to balance the
energy on both sides of the equation.
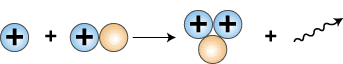
Release of gamma ray photon
Courtesy: McREL
|
Finally, as the last step, two helium-3 nuclei collide
to form helium-4, , and two protons.
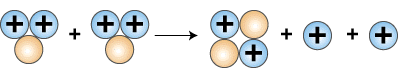
Formation of helium nucleus
Courtesy: McREL
|
The overall net reaction becomes:
nc
+ 2 g
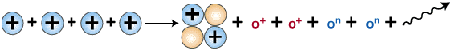
Solar hydrogen fusion yields helium and energy
Courtesy: McREL
|
The only energy production mentioned in the equation
above is the gamma rays, which are the original source of
the sun's radiated energy. They must eventually work their
way out of the core.
The hydrogen nuclei (protons) at the sun's core are
hydrogen atoms from which electrons have been ripped away
(ionized nuclei). The hot, rapidly moving protons are mixed
with an immense number of loose electrons. The positrons formed
in the first equation nearly instantaneously encounter their
sub-atomic anti-partnersthe electrons, instantly annihilating
each other and producing a flash of pure energy in the form
of gamma ray photons.
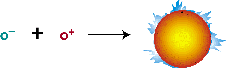
Annihilation of electron and positron
Courtesy: McREL
|
The positron and the electron both have mass (albeit
small). Their combined masses are destroyed completely and
turned into energy, according to the Einstein relationship
. Detailed calculations actually
show that mass is lost and converted to energy in each of
the nuclear reaction steps and these collective mass losses
account for the total energy output of the sun.
The scenario outlined above is called the proton-proton
chain. It is the most important process for producing the
sun's energy, although it is not the only set of reactions
that occur.
Other fusion reactions
At even higher temperatures inside the sun and other
aging stars, other nuclei undergo fusion reactions. These
reactions occur in layers, with the higher temperature layers
closer to the center. Some examples are given in the table
below.
|
Temperature |
| ~2 x
ÁK |
~5 x
ÁK |
~10 x
ÁK |
He burning occurs
g
g
g
g
|
C burning occurs
+ g
|
myriad reactions occur
+ g
+ g
+ g
+ g |
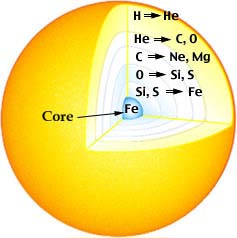
Nuclear Reaction Layers in an Aging Star
Courtesy: McREL
|
Reaction rates
It takes a given proton 14,000 million years to find
a "hot" partner. How does the sun's prodigious energy production
arise from the proton-proton chain when the reaction rate
is so low? The answer is that there are a stupendous number
of protons available in the sun. Based on the sun's luminosity
and the energy released per proton-proton chain event, the
number of core reactions occurring every second is calculated
to be about . The sun's mass
is being consumed at the astounding rate of
kg per second.

Courtesy: NASA
|
This mind-boggling number might seem alarming at first
glance. Is the sun in danger of running out of hydrogen? No,
absolutely not. Presently the mass of the sun is almost kg. In other words, the sun still
has a lot of hydrogen to work with. In fact, over the 4.5
billion years that the sun has shone, only about 0.03% of
its mass has been consumed. The sun is in the middle of its
life cycle, and will be heating and lighting the planets for
billions of years to come.
Fusion chemistry as described above forms the basis
of the Standard Solar Model, an explanation of the sun's composition
and functioning used by Genesis scientists in their design
of solar wind collection devices. The results of the analysis
of the samples of solar wind collected in the Genesis sample
return capsule will test the effectiveness of this model in
explaining the formation of the solar system. |
