Many cosmologists believe that the universe was created about 15 billion years ago with a cosmic explosion they nicknamed the Big Bang. This explosion produced an expanding cloud of the simplest known chemical elements: hydrogen (H) and helium (He). See Figure 1.
|
|
Figure 1. Atomic model of the two simplest elements, Hydrogen and Helium
Note. From U.S. Nuclear regulatory commission. Electron [ Online] |
In certain places there happened to be higher concentrations of this gas mixture than in others. Where gas particles were close together, their gravity pulled them even closer. These local concentrations of hydrogen and helium led to the growth of the first generation of stars. But they looked more like clouds of gas than adult stars, and they did not release any light. See Figure 2.
|
Figure 2. The birth of stars in the M16 Eagle Nebula
Note. From Hester, J. & Scowen, P. (1995, November). Star-Birth Clouds • M16 [ Online] |
As the young stars grew, their gravity increased, allowing them to continue pulling in hydrogen and helium. With a gain in material, the pressure and temperature in the center of the gas clouds increased dramatically. This pressure started the process of nuclear fusion. This is how other chemical elements were formed inside the first stars (see Figure 3).
|
Figure 3. Nuclear Fusion of Hydrogen into Helium
Note. From Cornell University Instructional Web Server Information
and Services Hydrogen Burning [ Online].
|
Because nuclear fusion is a process that releases a lot of energy, the growing stars soon released light and heat. At this point in development, the balls of gas began to shine. They became recognizable as stars.
As the stars aged, the hydrogen, helium and other chemical elements used to create energy in their centers were used up. The stars began to cool, as though their nuclear furnaces had been turned off. As the outer layers of gas continued to cool down, they collapsed toward the center. What happened next depended on the size of the star.
In some cases, the collapsing stars exploded violently, becoming supernovas. The energized material in the expanding supernovas rapidly created heavier chemical elements. Much of the material that made up these stars was spewed into space (see Figure 4).
|
Figure 4. Expanding explosion debris from Supernova 1987A
Note. From Pun, C.S.J., Kirshner, R.P., & NASA (1997, January).
Photo No. STScI-PRC97-03 [ Online]. |
In other cases, the dying stars slowly released their contents into space. Like the supernovas, the end result was the same. The space between surviving stars was enriched with heavier chemical elements as well as the simpler hydrogen and helium that had made up most of the dying stars. From the heavier elements, many of the gaseous atoms solidified to form small grains or crystals that appeared like dust floating in the cold of outer space.
These processes of the death of stars and the formation of new stars and small dust grains happened again and again. Each successive generation of stars had higher amounts of the heavier chemical elements formed in the previous generation. The composition of newer stars was different from that of older stars.
Our star, the sun, belongs to the generation of stars created 4.6 billion years ago. A cloud of gas, dust, and frozen ice from between existing stars collapsed to form a nebula. This nebula spun slowly. Most fell to disk and then moved inward to form the sun. A small fraction of the material in the disk formed solids which bumped into each other, stuck and grew larger. As they grew larger, their gravity increased. These larger particles were able to attract even more dust and ice, increasing in size to become the cores of planets, moons or asteroids. Some drifted away from the sun to become comets (see Figure 5).
Figure 5. Protoplanetary disk around a developing star
Note. From Encarta 98 Encyclopedia Protoplanetary Disk
Since all nine planets formed from the same material, are they the same? They are not. Mercury and Venus, being closest to the sun, are both much hotter than the Earth, but Mercury has no atmosphere while Venus is covered with thick, poisonous clouds. Earth seems to be the only one of the planets hospitable to life. What makes the planets so different from each other? (See Figure 6.)
Figure 6. The three inner planets: Mercury, Venus, Earth
Note.
From California Institute of Technology (1995). Mercury, Venus, Earth [Online].
Cosmologists have evidence that the planets, moons, and even asteroids differ in the amounts of the various chemical elements from which they were made. These differences may provide scientists with clues into how the solar nebula of swirling gas, dust and ice formed into planets. They have made models of the process of planet formation. To best test these models, they need to know the starting conditions. What chemical elements were present in the original solar nebula?
The sun still contains most of the material of the original solar nebula. Its internal nuclear reactions have modified the material at the sun’s core. However, the surface layers, which have not mixed with the core in its present state, have quite accurately preserved the original nebular composition.
For practical reasons, we cannot send a spacecraft to the sun to pick up samples for analysis. However, the sun shoots out streams of its outer material, which we call solar wind. The Genesis spacecraft will collect samples of these chemicals and return them to Earth for scientists to analyze. These studies will enrich our understanding of the formation of the planets, their moons, and the asteroids (see Figure 7).
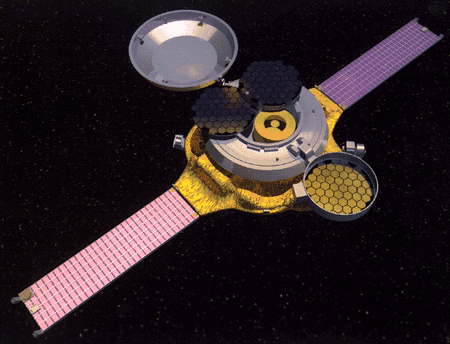 |
Figure 7. The Genesis module with collectors deployed |
|
