|


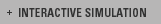


|
 |
 |
  |
|
 |
This science module is about the characteristics of chemical
elements and the processes of posing and answering questions
that led to the development of the periodic table. If you're
using Genesis science modules for the first time, read the User's
Guide thoroughly before you begin. (View User's Guide as
a PDF.)
The Portable Document Format (PDF) is used to distribute
fully formatted, print-quality documents electronically.
The following information is available to view and print
as a PDF file with Adobe's Acrobat reader. To install the
FREE Adobe Acrobat Reader, visit the Adobe
Web site.
Take a look at other science
modules available. All technical terms in the science
modules are compiled in the Glossary
for easy access.
Technology
Applications are available for this module.
|
|
|
|
|
Adobe's
Acrobat Reader©

The
Portable Document Format (PDF) is used to distribute
fully formatted, print-quality documents. |
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
|
| |
|
 |
| Use the Student
Activity, The
Search for Critical Questions, to create interest in learning
more about solving problems by asking questions. The activity
starts with students attempting to predict the characteristics
of pieces missing from a jigsaw puzzle. It concludes with an
examination of the questions that were formulated by lab groups
as they searched for understanding. |
|
 |
Curriculum
Connections
National Standards Addressed
National
Science Education Standards
Grades 9-12
Science as Inquiry |
- Abilities necessary to do scientific inquiry
- Understandings about scientific inquiry
|
| Physical Science |
- Conservation of energy and the increase in disorder
- Interactions of energy and matter
|
Science and
Technology
|
- Abilities of technological Design
- Understandings about science and technology
|
| Science in Personal
and Social Perspective |
- Natural and human-induced hazards
- Science and technology in local, national, and global
challenges
|
| History and
Nature of Science |
- Science as a human endeavor
- Nature of scientific knowledge
- Historical perspectives
|
|
|
|
|
|
|
 |
|
|
|
|
 |
In the activities to come, the
teacher's instructional role is socratic. Through effective questioning,
students are prompted to examine their logic as they attempt
to solve two major mysteries— Mendeleev's landmark development
of the Periodic Table of Elements, and the Genesis mission scientists'
quest for elemental and isotopic abundance clues to the origin
of the solar system.
 Use
the activity, Exploration of
a Problem: Making Sense of the Elements, to generate
discussion, leading students to examine some of their
basic assumptions. This activity offers the teacher
a snapshot of the class's present level of understanding
about elements and their chemical characteristics. It
is up to the discretion of the teacher to determine
how much review or initial instruction of chemistry
concepts is appropriate at this time. Use
the activity, Exploration of
a Problem: Making Sense of the Elements, to generate
discussion, leading students to examine some of their
basic assumptions. This activity offers the teacher
a snapshot of the class's present level of understanding
about elements and their chemical characteristics. It
is up to the discretion of the teacher to determine
how much review or initial instruction of chemistry
concepts is appropriate at this time.
|
|
|
|
|
|
|
|
|
|
|
| Models corresponding to real events
and objects help scientists understand and explain how things work.
These explanations also generate logic-based models through incorporation
of new findings. Terms such as "model" and "theory" become easier
for students to understand as they construct a model and offer
explanations
based on critical questioning and applications of mathematical
and logical concepts. |
- During this more formal encounter with the investigative
process, students in lab groups ask questions, construct models,
make observations, and read and discuss text materials. Using
the student activity, Development of a Model: Analyzing Elemental
Abundance, each student records data, conducts analyses, and
interprets relationships between
 evidence
and decision making. evidence
and decision making.
- Teachers may introduce technical vocabulary at this point
in the learning cycle. The student text, Atoms, Elements, and
Isotopes, can be used at this point, or later in the process,
wherever deemed appropriate by the teacher.
|
|
|
|
|
 |
|
| Development of a Model: Analyzing
Elemental Abundance |
 |
Teacher
Guide |
Development of a Model: Analyzing Elemental
Abundances on Earth |
 |
Student
Activity |
|
| |
 |
Students interact with peers in
order to accomplish many of the tasks in the sections above.
Each activity contains lab work done in groups, and preliminary
and summary discussions are held as a class.
Students are asked to individually synthesize
their knowledge by using what they have learned in group
activities and discussions to answer a series of related
questions in their laboratory notebook or in another format
determined by the teacher. |
 In
the activity, Development of a Model:
Analyzing Extraterrestrial Elemental Abundances, students
determine the origin of material from Antarctica, comparing
its oxygen isotope ratios to those of other identified samples.
Students integrate all they have learned about asking questions
and creating graphs to analyze data on these samples. The activity
concludes with the class holding a public discussion in which
lab groups argue for further support of this type of research. In
the activity, Development of a Model:
Analyzing Extraterrestrial Elemental Abundances, students
determine the origin of material from Antarctica, comparing
its oxygen isotope ratios to those of other identified samples.
Students integrate all they have learned about asking questions
and creating graphs to analyze data on these samples. The activity
concludes with the class holding a public discussion in which
lab groups argue for further support of this type of research.
|
|
|
|
|
 |
|
|
| |
 |
| Use the final activity, Connecting
Models and Critical Questions, to assess students' abilities
to search for patterns in tables of data, to create mathematical
models, and to communicate their findings with their peers.
|
- In Connecting
Models and Critical Questions, students initially
meet in lab groups to discuss information provided about
a subset of the elements. They finish the assessment
individually, creating a mathematical model to explain
differences in chemical reactivity among these elements,
analyzing the process of developing this model, interpolating
from their model the characteristics of a hypothetical
element, and planning a presentation to their peers on
their model.
|
|
|
|
|
 |
|
|
|
|
 |
|
|
|
|
|
|
| |
|
 |
|
| |
|
|
| Principal Developer: |
Greg
Rawls, Genesis Education Outreach Manager
|
| Contributing Writers: |
Jacinta Behne
Dr. Martha Henry
Jeff Johnson
Alice Krueger
Greg Rawls
Dr. John Sutton
|
| Technical Editor: |
Jacinta Behne, McREL
|
|
| Special thanks to the following reviewers: |
Dr. Donna Bogner, Wichita State University
Dr. Don Burnett, California Institute of Technology
Dr. Marcia Neugebauer, Jet Propulsion Laboratory
Dr. Don Rapp, Jet Propulsion Laboratory
Dr. Dorothy Woolum, Jet Propulsion Laboratory
|
| Graphics provided by: |
Viewmark, Inc.
Dr. John Sutton, McREL
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
